Back Adrenalien Afrikaans أدرينالين Arabic Adrenalina AST Adrenalin Azerbaijani اپینفرین AZB Адрэналін Byelorussian Адреналин Bulgarian एड्रेनेलिन Bihari অ্যাড্রেনালিন Bengali/Bangla Adrenalin BS
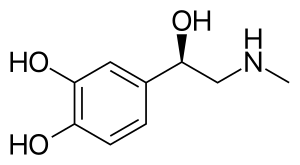
Adrenaline, also known as epinephrine, is a hormone and medication[7][8] which is involved in regulating visceral functions (e.g., respiration).[7][9] It appears as a white microcrystalline granule.[10] Adrenaline is normally produced by the adrenal glands and by a small number of neurons in the medulla oblongata.[11] It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output by acting on the SA node,[12] pupil dilation response, and blood sugar level.[13][14] It does this by binding to alpha and beta receptors.[14] It is found in many animals, including humans, and some single-celled organisms.[15][16] It has also been isolated from the plant Scoparia dulcis found in Northern Vietnam.[17]
- ^ Andersen AM (1975). "Structural Studies of Metabolic Products of Dopamine. III. Crystal and Molecular Structure of (−)-Adrenaline". Acta Chem. Scand. 29b (2): 239–244. doi:10.3891/acta.chem.scand.29b-0239. PMID 1136652.
- ^ El-Bahr SM, Kahlbacher H, Patzl M, Palme RG (May 2006). "Binding and clearance of radioactive adrenaline and noradrenaline in sheep blood". Veterinary Research Communications. 30 (4). Springer Science and Business Media LLC: 423–32. doi:10.1007/s11259-006-3244-1. PMID 16502110. S2CID 9054777.
- ^ Franksson G, Anggård E (13 March 2009). "The plasma protein binding of amphetamine, catecholamines and related compounds". Acta Pharmacologica et Toxicologica. 28 (3). Wiley: 209–14. doi:10.1111/j.1600-0773.1970.tb00546.x. PMID 5468075.
- ^ Peaston RT, Weinkove C (January 2004). "Measurement of catecholamines and their metabolites". Annals of Clinical Biochemistry. 41 (Pt 1). SAGE Publications: 17–38. doi:10.1258/000456304322664663. PMID 14713382. S2CID 2330329.
- ^ Cite error: The named reference
AHFS2015was invoked but never defined (see the help page). - ^ Hummel MD (2012). "Emergency Medications". In Pollak AN (ed.). Nancy Caroline's Emergency Care in the Streets (7th ed.). Burlington: Jones & Bartlett Learning. p. 557. ISBN 9781449645861. Archived from the original on 8 September 2017.
- ^ a b Lieberman M, Marks A, Peet A (2013). Marks' Basic Medical Biochemistry: A Clinical Approach (4th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 175. ISBN 9781608315727.
- ^ "Adrenaline". 21 August 2015.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 157. ISBN 9780071481274.
Epinephrine occurs in only a small number of central neurons, all located in the medulla. Epinephrine is involved in visceral functions, such as the control of respiration. It is also produced by the adrenal medulla.
- ^ Larrañaga M (2016). Hawley's Condensed Chemical Dictionary. New Jersey: John Wiley & Sons, Incorporated. p. 561.
- ^ "Adrenaline: physiology and pharmacology | DermNet". dermnetnz.org. Retrieved 20 March 2023.
- ^ Brown HF, DiFrancesco D, Noble SJ (July 1979). "How does adrenaline accelerate the heart?". Nature. 280 (5719): 235–236. Bibcode:1979Natur.280..235B. doi:10.1038/280235a0. PMID 450140. S2CID 4350616.
- ^ Bell DR (2009). Medical physiology : principles for clinical medicine (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 312. ISBN 9780781768528.
- ^ a b Khurana I (2008). Essentials of Medical Physiology. Elsevier India. p. 460. ISBN 9788131215661.
- ^ Buckley E (2013). Venomous Animals and Their Venoms: Venomous Vertebrates. Elsevier. p. 478. ISBN 9781483262888.
- ^ Animal Physiology: Adaptation and Environment (5th ed.). Cambridge University Press. 1997. p. 510. ISBN 9781107268500.
- ^ Phan MG, Phan TS, Matsunami K, Otsuka H (April 2006). "Chemical and biological evaluation on scopadulane-type diterpenoids from Scoparia dulcis of Vietnamese origin". Chemical & Pharmaceutical Bulletin. 54 (4): 546–549. doi:10.1248/cpb.54.546. PMID 16595962.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search