
Back هيم Arabic হিম Bengali/Bangla Hem BS Grup hemo Catalan ھیم CKB Hem Czech Häme (Stoffgruppe) German Αίμη Greek Hemo Esperanto Hemo Spanish

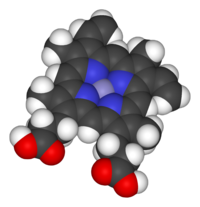
Heme (American English), or haem (Commonwealth English, both pronounced /hi:m/ HEEM), is a ring-shaped iron-containing molecular component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 vinyl and 2 propionic acid side chains.[1] Heme is biosynthesized in both the bone marrow and the liver.[2]
Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen moiety. Reactions include oxidative metabolism (cytochrome c oxidase, succinate dehydrogenase), xenobiotic detoxification via cytochrome P450 pathways (including metabolism of some drugs), gas sensing (guanyl cyclases, nitric oxide synthase), and microRNA processing (DGCR8).[3][4]
Heme is a coordination complex "consisting of an iron ion coordinated to a tetrapyrrole acting as a tetradentate ligand, and to one or two axial ligands".[5] The definition is loose, and many depictions omit the axial ligands.[6] Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used[7] and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase.[8][9]
The word haem is derived from Greek αἷμα haima 'blood'.
- ^ Hodgson E, Roe RM, Mailman RB, Chambers JE, eds. (2015). "H". Dictionary of Toxicology (3rd ed.). Academic Press. pp. 173–184. doi:10.1016/B978-0-12-420169-9.00008-4. ISBN 978-0-12-420169-9. Retrieved 2024-02-21.
- ^ Bloomer JR (1998). "Liver metabolism of porphyrins and haem". Journal of Gastroenterology and Hepatology. 13 (3): 324–329. doi:10.1111/j.1440-1746.1998.01548.x. PMID 9570250. S2CID 25224821.
- ^ Dutt S, Hamza I, Bartnikas TB (2022-08-22). "Molecular Mechanisms of Iron and Heme Metabolism". Annual Review of Nutrition. 42 (1): 311–335. doi:10.1146/annurev-nutr-062320-112625. ISSN 0199-9885. PMC 9398995. PMID 35508203.
- ^ Ogun AS, Joy NV, Valentine M (2024), "Biochemistry, Heme Synthesis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30726014, retrieved 2024-02-22
- ^ Chemistry IU (2009). "Hemes (heme derivatives)". IUPAC Compendium of Chemical Terminology. IUPAC. doi:10.1351/goldbook.H02773. ISBN 978-0-9678550-9-7. Archived from the original on 22 August 2017. Retrieved 28 April 2018.
- ^ A standard biochemistry text defines heme as the "iron-porphyrin prosthetic group of heme proteins"(Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.)
- ^ Poulos TL (2014-04-09). "Heme Enzyme Structure and Function". Chemical Reviews. 114 (7): 3919–3962. doi:10.1021/cr400415k. ISSN 0009-2665. PMC 3981943. PMID 24400737.
- ^ Paoli M (2002). "Structure-function relationships in heme-proteins" (PDF). DNA Cell Biol. 21 (4): 271–280. doi:10.1089/104454902753759690. hdl:20.500.11820/67200894-eb9f-47a2-9542-02877d41fdd7. PMID 12042067. S2CID 12806393. Archived (PDF) from the original on 2018-07-24.
- ^ Alderton W (2001). "Nitric oxide synthases: structure, function and inhibition". Biochem. J. 357 (3): 593–615. doi:10.1042/bj3570593. PMC 1221991. PMID 11463332.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search