
Back Aromatisiteit (chemie) Afrikaans عطرية Arabic Aromatiklik termini Azerbaijani অ্যারোম্যাটিসিটি Bengali/Bangla Aromatičnost BS Aromaticitat Catalan Aromaticita Czech Ароматлăх CV Aromatizität German Αρωματικότητα Greek
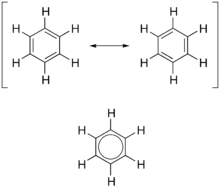
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855.[1] There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds.
Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance.[2][3][4] This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to give rise to six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.
- ^ A. W. Hofmann (1855). "On Insolinic Acid". Proceedings of the Royal Society. 8: 1–3. doi:10.1098/rspl.1856.0002.
- ^ Schleyer, Paul von Ragué (2001). "Introduction: Aromaticity". Chemical Reviews. 101 (5): 1115–8. doi:10.1021/cr0103221. PMID 11749368.
- ^ A. T. Balaban, P. v. R. Schleyer and H. S. Rzepa (2005). "Crocker, Not Armit and Robinson, Begat the Six Aromatic Electrons". Chemical Reviews. 105 (10): 3436–3447. doi:10.1021/cr0300946.
- ^ Schleyer, Paul von Ragué (2005). "Introduction: Delocalization Pi and Sigma". Chemical Reviews. 105 (10): 3433. doi:10.1021/cr030095y.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search