
Back نواة متكئة Arabic نوايه متكئه ARZ Nucleus accumbens Czech Nucleus accumbens German Επικλινής πυρήνας Greek Núcleo accumbens Spanish هسته آکومبنس Persian Noyau accumbens French גרעין האקומבנס HE Nucleus accumbens Italian
| Nucleus accumbens | |
|---|---|
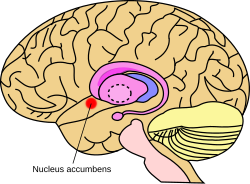 Approximate location of the nucleus accumbens in the brain | |
 Nucleus accumbens of the mouse brain | |
| Details | |
| Part of | Mesolimbic pathway Basal ganglia (Ventral striatum) |
| Parts | Nucleus accumbens shell Nucleus accumbens core |
| Identifiers | |
| Latin | nucleus accumbens septi |
| Acronym(s) | NAc or NAcc |
| MeSH | D009714 |
| NeuroNames | 277 |
| NeuroLex ID | birnlex_727 |
| TA98 | A14.1.09.440 |
| TA2 | 5558 |
| FMA | 61889 |
| Anatomical terms of neuroanatomy | |
The nucleus accumbens (NAc or NAcc; also known as the accumbens nucleus, or formerly as the nucleus accumbens septi, Latin for 'nucleus adjacent to the septum') is a region in the basal forebrain rostral to the preoptic area of the hypothalamus.[1] The nucleus accumbens and the olfactory tubercle collectively form the ventral striatum. The ventral striatum and dorsal striatum collectively form the striatum, which is the main component of the basal ganglia.[2] The dopaminergic neurons of the mesolimbic pathway project onto the GABAergic medium spiny neurons of the nucleus accumbens and olfactory tubercle.[3][4] Each cerebral hemisphere has its own nucleus accumbens, which can be divided into two structures: the nucleus accumbens core and the nucleus accumbens shell. These substructures have different morphology and functions.
Different NAcc subregions (core vs shell) and neuron subpopulations within each region (D1-type vs D2-type medium spiny neurons) are responsible for different cognitive functions.[5][6] As a whole, the nucleus accumbens has a significant role in the cognitive processing of motivation, aversion, reward (i.e., incentive salience, pleasure, and positive reinforcement), and reinforcement learning (e.g., Pavlovian-instrumental transfer);[4][7][8][9][10] hence, it has a significant role in addiction.[4][8] In addition, part of the nucleus accumbens core is centrally involved in the induction of slow-wave sleep.[11][12][13][14] The nucleus accumbens plays a lesser role in processing fear (a form of aversion), impulsivity, and the placebo effect.[15][16][17] It is involved in the encoding of new motor programs as well.[4]
- ^ Carlson NR (2013). Physiology of Behavior (11th ed.). Boston: Pearson. [page needed]
- ^ Nucleus Accumbens
- ^ Ikemoto S (November 2010). "Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory". Neuroscience and Biobehavioral Reviews. 35 (2): 129–50. doi:10.1016/j.neubiorev.2010.02.001. PMC 2894302. PMID 20149820.
Recent studies on intracranial self-administration of neurochemicals (drugs) found that rats learn to self-administer various drugs into the mesolimbic dopamine structures–the posterior ventral tegmental area, medial shell nucleus accumbens and medial olfactory tubercle. ... In the 1970s it was recognized that the olfactory tubercle contains a striatal component, which is filled with GABAergic medium spiny neurons receiving glutamatergic inputs form cortical regions and dopaminergic inputs from the VTA and projecting to the ventral pallidum just like the nucleus accumbens
Figure 3: The ventral striatum and self-administration of amphetamine - ^ a b c d Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–148, 367, 376. ISBN 978-0-07-148127-4.
VTA DA neurons play a critical role in motivation, reward-related behavior (Chapter 15), attention, and multiple forms of memory. This organization of the DA system, wide projection from a limited number of cell bodies, permits coordinated responses to potent new rewards. Thus, acting in diverse terminal fields, dopamine confers motivational salience ("wanting") on the reward itself or associated cues (nucleus accumbens shell region), updates the value placed on different goals in light of this new experience (orbital prefrontal cortex), helps consolidate multiple forms of memory (amygdala and hippocampus), and encodes new motor programs that will facilitate obtaining this reward in the future (nucleus accumbens core region and dorsal striatum). In this example, dopamine modulates the processing of sensorimotor information in diverse neural circuits to maximize the ability of the organism to obtain future rewards. ...
The brain reward circuitry that is targeted by addictive drugs normally mediates the pleasure and strengthening of behaviors associated with natural reinforcers, such as food, water, and sexual contact. Dopamine neurons in the VTA are activated by food and water, and dopamine release in the NAc is stimulated by the presence of natural reinforcers, such as food, water, or a sexual partner. ...
The NAc and VTA are central components of the circuitry underlying reward and memory of reward. As previously mentioned, the activity of dopaminergic neurons in the VTA appears to be linked to reward prediction. The NAc is involved in learning associated with reinforcement and the modulation of motoric responses to stimuli that satisfy internal homeostatic needs. The shell of the NAc appears to be particularly important to initial drug actions within reward circuitry; addictive drugs appear to have a greater effect on dopamine release in the shell than in the core of the NAc. - ^ Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM (August 2015). "Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation". The Journal of Neuroscience. 35 (33): 11572–82. doi:10.1523/JNEUROSCI.2344-15.2015. PMC 4540796. PMID 26290234.
Here, we have found that real-time dopamine release within the nucleus accumbens (a primary target of midbrain dopamine neurons) strikingly varies between core and shell subregions. In the core, dopamine dynamics are consistent with learning-based theories (such as reward prediction error) whereas in the shell, dopamine is consistent with motivation-based theories (e.g., incentive salience).
- ^ Cite error: The named reference
Reward and aversion MSNswas invoked but never defined (see the help page). - ^ Wenzel JM, Rauscher NA, Cheer JF, Oleson EB (January 2015). "A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature". ACS Chemical Neuroscience. 6 (1): 16–26. doi:10.1021/cn500255p. PMC 5820768. PMID 25491156.
Thus, fear-evoking stimuli are capable of differentially altering phasic dopamine transmission across NAcc subregions. The authors propose that the observed enhancement in NAcc shell dopamine likely reflects general motivational salience, perhaps due to relief from a CS-induced fear state when the US (foot shock) is not delivered. This reasoning is supported by a report from Budygin and colleagues112 showing that, in anesthetized rats, the termination of tail pinch results in augmented dopamine release in the shell.
- ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 266. ISBN 978-0-07-148127-4.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ^ Cite error: The named reference
Mesolimbic dopamine in motivation and PITwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Learning+Motivation in PIT - neural mechanisms reviewwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Zinc & sleep 2017 reviewwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Nucleus accumbens - Slow-wave sleepwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Slow-wave sleep and NAcc core A2AR neurons - Sept 2017 primary sourcewas invoked but never defined (see the help page). - ^ Cite error: The named reference
Striatal A2AR neurons and sleep - Oct 2017 primary sourcewas invoked but never defined (see the help page). - ^ Schwienbacher I, Fendt M, Richardson R, Schnitzler HU (November 2004). "Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats". Brain Research. 1027 (1–2): 87–93. doi:10.1016/j.brainres.2004.08.037. PMID 15494160. S2CID 18338111.
- ^ Zubieta JK, Stohler CS (March 2009). "Neurobiological mechanisms of placebo responses". Annals of the New York Academy of Sciences. 1156 (1): 198–210. Bibcode:2009NYASA1156..198Z. doi:10.1111/j.1749-6632.2009.04424.x. PMC 3073412. PMID 19338509.
- ^ Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y (December 2010). "Nucleus accumbens and impulsivity". Progress in Neurobiology. 92 (4): 533–57. doi:10.1016/j.pneurobio.2010.08.007. PMID 20831892. S2CID 16964212.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search