
Back فينوباربيتال Arabic Fenobarbital Catalan Fenobarbital Czech Ffenobarbital Welsh Phenobarbital Danish Phenobarbital German Φαινοβαρβιτάλη Greek Fenobarbital Spanish Fenobarbital Basque فنوباربیتال Persian
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Luminal, Sezaby |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682007 |
| Pregnancy category |
|
| Dependence liability | Low[1] |
| Routes of administration | By mouth, rectal, parenteral |
| Drug class | Barbiturate |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | >95% |
| Protein binding | 20 to 45% |
| Metabolism | Liver (mostly CYP2C19) |
| Onset of action | within 5 min (IV) and 30 min (PO)[4] |
| Elimination half-life | 53 to 118 hours |
| Duration of action | 4 hrs[4] to 2 days[5] |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.007 |
| Chemical and physical data | |
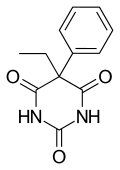
| Formula | C12H12N2O3 |
| Molar mass | 232.239 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type.[4] It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries.[6] In the developed world, it is commonly used to treat seizures in young children,[7] while other medications are generally used in older children and adults.[8] In developed countries it is used for veterinary purposes.[9] It may be used intravenously, injected into a muscle, or taken by mouth.[4] The injectable form may be used to treat status epilepticus.[4] Phenobarbital is occasionally used to treat trouble sleeping, anxiety, and drug withdrawal and to help with surgery.[4] It usually begins working within five minutes when used intravenously and half an hour when administered by mouth.[4] Its effects last for between four hours and two days.[4][5]
Side effects include a decreased level of consciousness along with a decreased effort to breathe.[4] There is concern about both abuse and withdrawal following long-term use.[4] It may also increase the risk of suicide.[4] It is pregnancy category B or D (depending on how it is taken) in the United States and category D in Australia, meaning that it may cause harm when taken by pregnant women.[4][10] If used during breastfeeding it may result in drowsiness in the baby.[11] A lower dose is recommended in those with poor liver or kidney function, as well as elderly people.[4] Phenobarbital, like other barbiturates works by increasing the activity of the inhibitory neurotransmitter GABA.[4]
Phenobarbital was discovered in 1912 and is the oldest still commonly used anti-seizure medication.[12][13] It is on the World Health Organization's List of Essential Medicines.[14]
- ^ Bassert JM (2017). McCurnin's Clinical Textbook for Veterinary Technicians - E-Book. Elsevier Health Sciences. p. 955. ISBN 9780323496407.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ "Sezaby- phenobarbital sodium injection". DailyMed. 6 January 2023. Retrieved 21 January 2023.
- ^ a b c d e f g h i j k l m n "Phenobarbital". The American Society of Health-System Pharmacists. Archived from the original on 2015-09-06. Retrieved Aug 14, 2015.
- ^ a b Marx JA (2010). Rosen's emergency medicine : concepts and clinical practice (7 ed.). Philadelphia: Mosby/Elsevier. p. 1352. ISBN 978-0-323-05472-0. Archived from the original on 2016-03-05.
- ^ Ilangaratne NB, Mannakkara NN, Bell GS, Sander JW (December 2012). "Phenobarbital: missing in action". Bulletin of the World Health Organization. 90 (12): 871–871A. doi:10.2471/BLT.12.113183. PMC 3524964. PMID 23284189.
- ^ Brodie MJ, Kwan P (December 2012). "Current position of phenobarbital in epilepsy and its future". Epilepsia. 53 (Suppl 8): 40–46. doi:10.1111/epi.12027. PMID 23205961. S2CID 25553143.
- ^ National Clinical Guideline Centre (UK) (January 2012). "The Epilepsies: The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care: Pharmacological Update of Clinical Guideline 20". National Institute for Health and Clinical Excellence: Guidance. London: Royal College of Physicians (UK). PMID 25340221 – via PubMed.
- ^ Thomas WB (2003). "Seizures and narcolepsy". In Dewey CW (ed.). A Practical Guide to Canine and Feline Neurology. Ames, Iowa: Iowa State Press. ISBN 978-0-8138-1249-6.
- ^ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Archived from the original on 8 April 2014. Retrieved 22 April 2014.
- ^ "Phenobarbital use while Breastfeeding". 2013. Archived from the original on 8 September 2015. Retrieved 14 August 2015.
- ^ Brenner GM, Stevens CW (2013). "Antiepileptic Drugs". Pharmacology (4th ed.). Philadelphia, PA: Elsevier/Saunders. p. 204. ISBN 978-1-4557-0278-7. Archived from the original on 2017-09-04.
- ^ Engel J (2008). Epilepsy : a comprehensive textbook (2nd ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 1431. ISBN 978-0-7817-5777-5. Archived from the original on 2016-03-05.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search