Back إبينفرين (دواء) Arabic Epineffrin Welsh Epinefrina (medicación) Spanish Էպինեֆրին (դեղամիջոց) Armenian 에피네프린 (약물) Korean Epinefrin (ilaç) Turkish Epinephrine (dược phẩm) Vietnamese 药物用肾上腺素 WUU 藥物用腎上腺素 Chinese
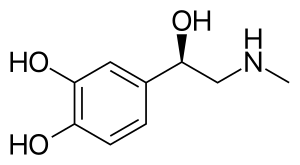
Epinephrine, also known as adrenaline, is a medication and hormone.[7][8] As a medication, it is used to treat several conditions, including anaphylaxis, cardiac arrest, asthma, and superficial bleeding.[5] Inhaled epinephrine may be used to improve the symptoms of croup.[9] It may also be used for asthma when other treatments are not effective.[5] It is given intravenously, by injection into a muscle, by inhalation, or by injection just under the skin.[5]
Common side effects include shakiness, anxiety, and sweating.[5] A fast heart rate and high blood pressure may occur.[5] Occasionally, it may result in an abnormal heart rhythm.[5] While the safety of its use during pregnancy and breastfeeding is unclear, the benefits to the mother must be taken into account.[5]
Epinephrine is normally produced by both the adrenal glands and a small number of neurons in the brain, where it acts as a neurotransmitter.[7][10] It plays an essential role in the fight-or-flight response by increasing blood flow to muscles, heart output, pupil dilation, and blood sugar.[11][12] Epinephrine does this through its effects on alpha and beta receptors.[12] It is found in many animals and some single-celled organisms,[13][14] but the medication is produced synthetically and is not harvested from animals.[15]
Jōkichi Takamine first isolated epinephrine in 1901, and it came into medical use in 1905.[16][17] It is on the World Health Organization's List of Essential Medicines.[18] It is available as a generic medication.[5] In 2021, it was the 221st most commonly prescribed medication in the United States, with more than 1 million prescriptions.[19][20]
- ^ Andersen AM (1975). "Structural Studies of Metabolic Products of Dopamine. III. Crystal and Molecular Structure of (−)-Adrenaline". Acta Chem. Scand. 29b (2): 239–244. doi:10.3891/acta.chem.scand.29b-0239. PMID 1136652.
- ^ El-Bahr SM, Kahlbacher H, Patzl M, Palme RG (May 2006). "Binding and clearance of radioactive adrenaline and noradrenaline in sheep blood". Veterinary Research Communications. 30 (4). Springer Science and Business Media LLC: 423–32. doi:10.1007/s11259-006-3244-1. PMID 16502110. S2CID 9054777.
- ^ Franksson G, Anggård E (13 March 2009). "The plasma protein binding of amphetamine, catecholamines and related compounds". Acta Pharmacologica et Toxicologica. 28 (3). Wiley: 209–14. doi:10.1111/j.1600-0773.1970.tb00546.x. PMID 5468075.
- ^ Peaston RT, Weinkove C (January 2004). "Measurement of catecholamines and their metabolites". Annals of Clinical Biochemistry. 41 (Pt 1). SAGE Publications: 17–38. doi:10.1258/000456304322664663. PMID 14713382. S2CID 2330329.
- ^ a b c d e f g h i "Epinephrine". The American Society of Health-System Pharmacists. Archived from the original on 6 September 2015. Retrieved 15 August 2015.
- ^ Hummel MD (2012). "Emergency Medications". In Pollak AN (ed.). Nancy Caroline's Emergency Care in the Streets (7th ed.). Burlington: Jones & Bartlett Learning. p. 557. ISBN 9781449645861. Archived from the original on 8 September 2017.
- ^ a b Lieberman M, Marks A, Peet A (2013). Marks' Basic Medical Biochemistry: A Clinical Approach (4 ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 175. ISBN 9781608315727. Archived from the original on 8 September 2017.
- ^ "(-)-adrenaline". Guide to Pharmacology. IUPS/BPS. Archived from the original on 1 September 2015. Retrieved 21 August 2015.
- ^ Everard ML (February 2009). "Acute bronchiolitis and croup". Pediatric Clinics of North America. 56 (1): 119–33, x–xi. doi:10.1016/j.pcl.2008.10.007. PMID 19135584.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 157. ISBN 9780071481274.
Epinephrine occurs in only a small number of central neurons, all located in the medulla. Epinephrine is involved in visceral functions, such as the control of respiration. It is also produced by the adrenal medulla.
- ^ Bell DR (2009). Medical physiology : principles for clinical medicine (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. p. 312. ISBN 9780781768528. Archived from the original on 8 September 2017.
- ^ a b Khurana (2008). Essentials of Medical Physiology. Elsevier India. p. 460. ISBN 9788131215661. Archived from the original on 8 September 2017.
- ^ Buckley E (2013). Venomous Animals and Their Venoms: Venomous Vertebrates. Elsevier. p. 478. ISBN 9781483262888. Archived from the original on 8 September 2017.
- ^ Animal Physiology: Adaptation and Environment (5 ed.). Cambridge University Press. 1997. p. 510. ISBN 9781107268500. Archived from the original on 8 September 2017.
- ^ "Epinephrine".
- ^ Wermuth CG (2008). The practice of medicinal chemistry (3 ed.). Amsterdam: Elsevier/Academic Press. p. 13. ISBN 9780080568775. Archived from the original on 8 September 2017.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 541. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Epinephrine - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search