
Back وارفارين Arabic وارفارین AZB ওয়ারফারিন Bengali/Bangla Warfarina Catalan Warffarin Welsh Warfarin Danish Warfarin German Βαρφαρίνη Greek Warfarina Spanish Varfariin Estonian
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈwɔːrfərɪn/ |
| Trade names | Coumadin, others[1][2][3] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682277 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 79–100% (by mouth)[7] |
| Protein binding | 99%[8] |
| Metabolism | Liver: CYP2C9, 2C19, 2C8, 2C18, 1A2 and 3A4[8] |
| Elimination half-life | 1 week (active half-life is 20-60 hours)[8] |
| Excretion | Kidney (92%)[8] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| PDB ligand | |
| ECHA InfoCard | 100.001.253 |
| Chemical and physical data | |
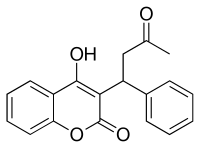
| Formula | C19H16O4 |
| Molar mass | 308.333 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Warfarin is an anticoagulant used as a medication under several brand names including Coumadin.[9] While the drug is described as a "blood thinner", it does not reduce viscosity but rather inhibits coagulation. Accordingly, it is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves.[9] Less commonly, it is used following ST-segment elevation myocardial infarction (STEMI) and orthopedic surgery.[9] It is usually taken by mouth, but may also be administered intravenously.[9]
The common side effect, a natural consequence of reduced clotting, is bleeding.[9] Less common side effects may include areas of tissue damage, and purple toes syndrome.[9] Use is not recommended during pregnancy.[9] The effects of warfarin are typically monitored by checking prothrombin time (INR) every one to four weeks.[9] Many other medications and dietary factors can interact with warfarin, either increasing or decreasing its effectiveness.[9][10] The effects of warfarin may be reversed with phytomenadione (vitamin K1), fresh frozen plasma, or prothrombin complex concentrate.[10]
Warfarin decreases blood clotting by blocking vitamin K epoxide reductase, an enzyme that reactivates vitamin K1.[10] Without sufficient active vitamin K1, the plasma concentrations of clotting factors II, VII, IX, and X are reduced and thus have decreased clotting ability.[10] The anticlotting protein C and protein S are also inhibited, but to a lesser degree.[10] Despite being labeled a vitamin K antagonist, warfarin does not antagonize the action of vitamin K1, but rather antagonizes vitamin K1 recycling, depleting active vitamin K1. A few days are required for full effect to occur, and these effects can last for up to five days.[9][11] Because the mechanism involves enzymes such as VKORC1, patients on warfarin with polymorphisms of the enzymes may require adjustments in therapy if the genetic variant that they have is more readily inhibited by warfarin, thus requiring lower doses.[12][13]
Warfarin first came into large-scale commercial use in 1948 as a rat poison.[14][15] It was formally approved as a medication to treat blood clots in humans by the U.S. Food and Drug Administration in 1954.[9] In 1955, warfarin's reputation as a safe and acceptable treatment was bolstered when President Dwight D. Eisenhower was treated with warfarin following a massive and highly publicized heart attack.[16] Eisenhower's treatment kickstarted a transformation in medicine whereby coronary artery disease, arterial plaques, and ischemic strokes were treated and protected against by using anticoagulants such as warfarin. It is on the World Health Organization's List of Essential Medicines.[17][18] Warfarin is available as a generic medication[19] and under many trade names.[1] In 2021, it was the 56th most commonly prescribed medication in the United States, with more than 11 million prescriptions.[20][21]
- ^ a b Cite error: The named reference
brandswas invoked but never defined (see the help page). - ^ Cite error: The named reference
brands2was invoked but never defined (see the help page). - ^ Cite error: The named reference
brands3was invoked but never defined (see the help page). - ^ "Warfarin Use During Pregnancy". Drugs.com. 4 September 2019. Archived from the original on 9 February 2018. Retrieved 7 February 2020.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "Coumadin- warfarin sodium tablet". DailyMed. Archived from the original on 26 December 2021. Retrieved 25 December 2021.
- ^ Holford NH (December 1986). "Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship". Clinical Pharmacokinetics. 11 (6): 483–504. doi:10.2165/00003088-198611060-00005. PMID 3542339. S2CID 92210077.
- ^ a b c d Cite error: The named reference
TGAwas invoked but never defined (see the help page). - ^ a b c d e f g h i j k "Warfarin sodium". The American Society of Health-System Pharmacists. 13 October 2022. Archived from the original on 12 June 2018. Retrieved 16 February 2023.
- ^ a b c d e Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G (February 2012). "Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines". Chest. 141 (2 Suppl): e44S–e88S. doi:10.1378/chest.11-2292. PMC 3278051. PMID 22315269.
- ^ Arcangelo VP, Peterson AM (2006). Pharmacotherapeutics for Advanced Practice: A Practical Approach. Lippincott Williams & Wilkins. p. 774. ISBN 978-0-7817-5784-3. Archived from the original on 18 September 2017.
- ^ Dasgupta A, Wahed A (2014). "Pharmacogenomics". Clinical Chemistry, Immunology and Laboratory Quality Control. pp. 353–362. doi:10.1016/B978-0-12-407821-5.00020-6. ISBN 978-0-12-407821-5.
- ^ "Warfarin". ScienceDirect. Archived from the original on 16 November 2021. Retrieved 16 November 2021.
- ^ Ravina E (2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 148. ISBN 978-3-527-32669-3. Archived from the original on 18 September 2017.
- ^ Cite error: The named reference
poisonwas invoked but never defined (see the help page). - ^ Lim GB (December 2017). "Milestone 2: Warfarin: from rat poison to clinical use". Nature Reviews. Cardiology. doi:10.1038/nrcardio.2017.172. PMID 29238065.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ British national formulary (69 ed.). British Medical Association. 2015. pp. 154–155. ISBN 978-0-85711-156-2.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Warfarin - Drug Usage Statistics". ClinCalc. Archived from the original on 13 April 2020. Retrieved 14 January 2024.
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search