Back دئوتراتد کولوروفرم AZB Cloroform deuterat Catalan Deuterokloroformo Basque دئوتراتد کلروفرم Persian Chloroforme deutéré French 重水素化クロロホルム Japanese Gedeutereerd chloroform Dutch Deuterisani hloroform Serbo-Croatian Deuterovaný chloroform Slovak Deuterisani hloroform Serbian
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
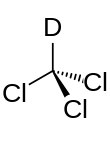
trichloro(deuterio)methane[1]
| |||
| Other names
Chloroform-d
Deuterochloroform | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1697633 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.011.585 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| UN number | 1888 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CDCl3 | |||
| Molar mass | 120.384 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | chloroform-like | ||
| Density | 1.500 g/cm3 | ||
| Melting point | −64 °C (−83 °F; 209 K) | ||
| Boiling point | 61 °C (142 °F; 334 K) | ||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| Danger | |||
| H302, H315, H319, H331, H336, H351, H361, H372, H373 | |||
| P201, P202, P260, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P311, P312, P314, P321, P330, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds
|
Chloroform | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Deuterated chloroform, also known as chloroform-d, is the organic compound with the formula CDCl3. Deuterated chloroform is a common solvent used in NMR spectroscopy.[2] The properties of CDCl3 and ordinary CHCl3 (chloroform) are virtually identical.
Deuterochloroform was first made in 1935 during the years of research on deuterium.[3]
- ^ "Chloroform-d".
- ^ Fulmer, Gregory R.; Miller, Alexander J. M.; Sherden, Nathaniel H.; Gottlieb, Hugo E.; Nudelman, Abraham; Stoltz, Brian M.; Bercaw, John E.; Goldberg, Karen I. (2010). "NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist" (PDF). Organometallics. 29 (9): 2176–2179. doi:10.1021/om100106e.
- ^ Chloroform-d (Deuteriochloroform), F. W. Breuer , J. Am. Chem. Soc. 1935, 57, 11, 2236–2237 (November 1, 1935) [1]
© MMXXIII Rich X Search. We shall prevail. All rights reserved. Rich X Search